Abstract
Background: Allogenic hematopoietic stem cell transplant (HSCT) is the potentially curative treatment in intermediate-high risk acute myeloid leukemia (AML). Relapse is the major cause of treatment failure and about 50% of AML patients relapse after the HSCT. Venetoclax is a B-cell lymphoma-2 (BCL-2) inhibitor that can restore activation of apoptosis in malignant cells. The FDA has approved venetoclax for the treatment of newly diagnosed adult AML patients unfit for intensive chemotherapy. We present a systemic review aimed to evaluate outcomes with venetoclax in patients with relapsed AML after HSCT.
Methods: We performed a literature search on 3 databases (Pubmed, Cochrane, and Clinicaltrials.gov) following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines using the Mesh terms "leukemia, myeloid, acute" OR "myelodysplastic syndromes" OR "hematopoietic stem cell transplantation" AND "venetoclax." After excluding duplicates and non-relevant articles, 161 studies including the case reports, review papers, and clinical trials were identified, and 7 studies were included in the analysis. Quality evaluation was done using the NIH quality assessment tool. Pooled analysis was done using the 'meta' package (Schwarzer et al, R programming language) and proportions with 95% confidence intervals (CI) were computed. The Inter-study heterogeneity among the studies was assessed using the Q statistic proposed by Cochrane and the I 2 index introduced by Higgins and Thompson.
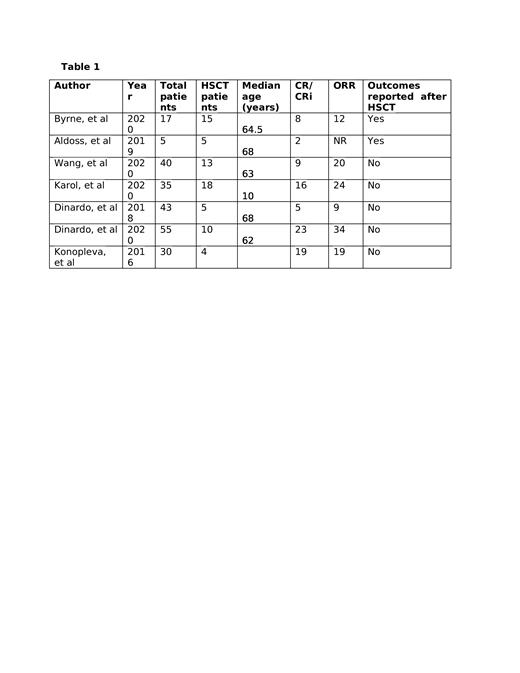
Results: Of 7 included studies, only 2 studies reported outcomes with the use of venetoclax in relapsed AML after HSCT and the rest of studies reported outcomes in all relapsed AML patients. (Table 1) Complete response (CR) with or without hematological recovery (CRi) was reported in 37% of patients (95% CI 24-52, I 2=75%, n=225). Morphologic leukemia free state (MLFS) was noted in 14% of patients (95% CI 0.08-0.22, I 2=31%, n=190) with an overall response rate (ORR) of 55% (95% CI 0.40-0.70, I 2=78%, n=220).
Conclusion: Venetoclax has showed promising results in relapsed AML, but there is very limited data evaluating the role of venetoclax after HSCT in relapsed AML. Venetoclax is increasingly used off-label after HSCT in relapsed AML and our findings highlight the need for prospective studies to evaluate the safety and efficacy of venetoclax in this patient population.
Anwer: GlaxoSmithKline: Research Funding; BMS / Celgene: Honoraria, Research Funding; Allogene Therapeutics: Research Funding; Janssen pharmaceutical: Honoraria, Research Funding. Abhyankar: Incyte/Therakos: Consultancy, Research Funding, Speakers Bureau. McGuirk: Allovir: Consultancy, Honoraria, Research Funding; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; EcoR1 Capital: Consultancy; Novartis: Research Funding; Bellicum Pharmaceuticals: Research Funding; Astelllas Pharma: Research Funding; Gamida Cell: Research Funding; Fresenius Biotech: Research Funding; Novartis: Research Funding; Kite/ Gilead: Consultancy, Honoraria, Other: travel accommodations, expense, Kite a Gilead company, Research Funding, Speakers Bureau; Pluristem Therapeutics: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal